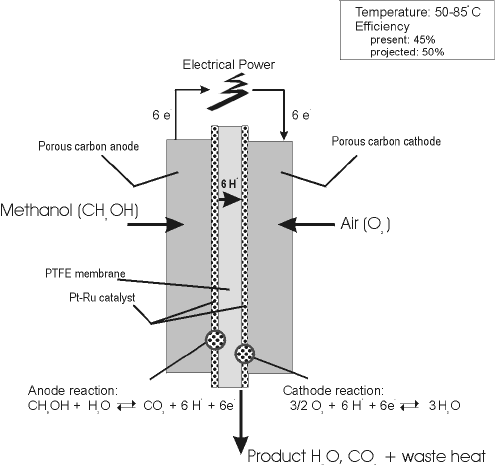
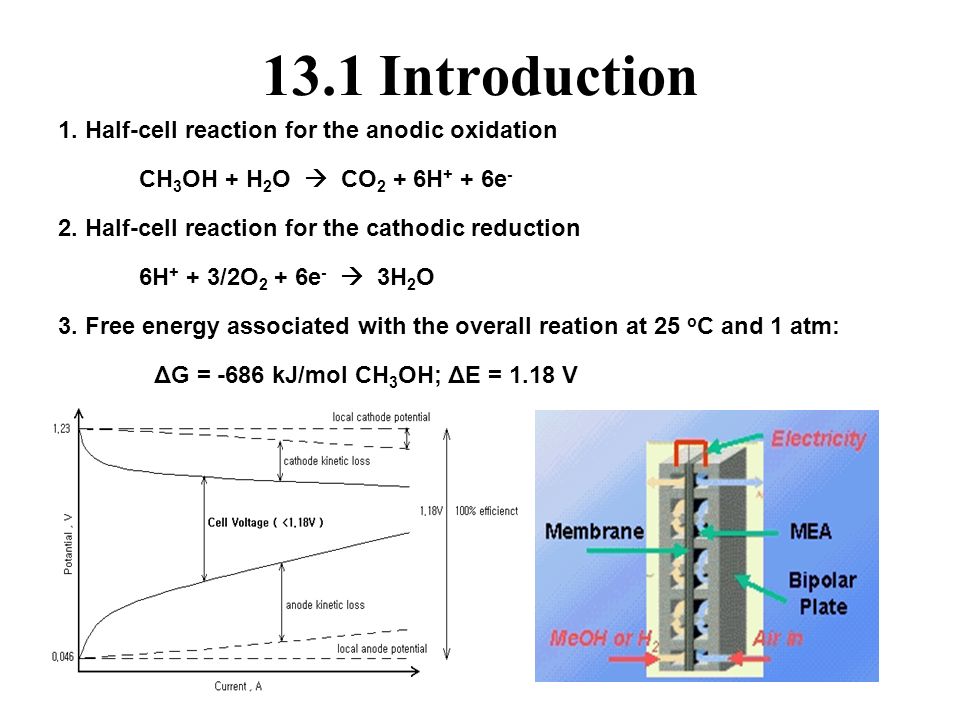
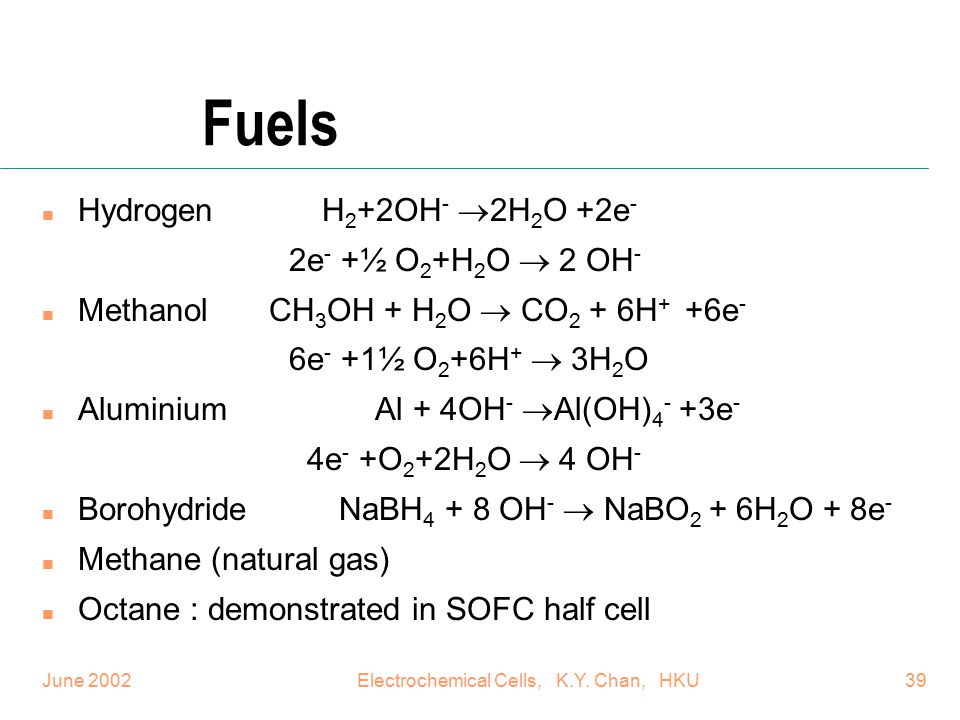
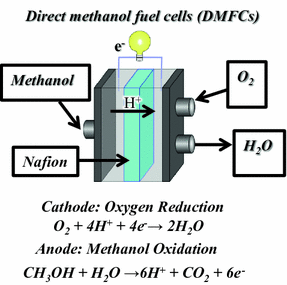
Methanol Fuel Cell Half Equations

About 3 in mass to carry the reactant into the cell.
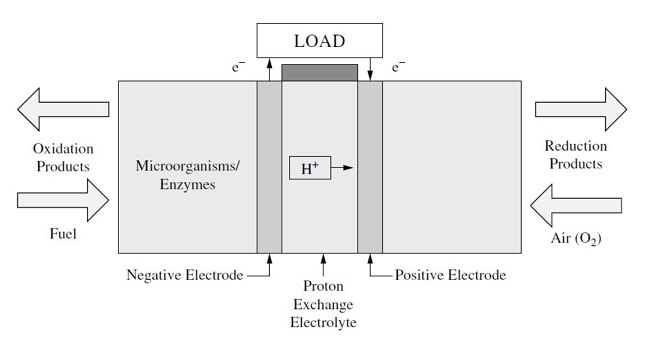
Methanol fuel cell half equations. For hydrogen oxygen fuel cell with reaction 2h 2 g o 2 g rarr 2 h 2 o l deltag f c h 2 o 237 2kj mol 1. Explain how the flow of ions allows for the operation of the fuel cell. Direct methanol fuel cell. O2 4h 4e 2h2o a write an equation for the complete combustion of methanol.
Bio ethanol based fuel cells may improve the well to wheel balance of this biofuel because of the increased conversion rate of the fuel cell compared to the internal combustion engine. Outline one advantage and one disadvantage of the methanol cell dmfc compared with a. The overall reaction in a hydrogen oxygen fuel cell is. I wrote ch3oh 1 5o2 co2 2h2o b deduce the half equation for the reaction that takes place at the positive electrode in a methanol fuel cell.
2h 2 4oh. At the negative electrode. Oxygen reacts at the negative electrode of a methanol fuel cell. Deduce the half cell equations occurring at each electrode during discharge.
Steam reforming of methanol at 250 deg c produces co 2 and h 2 along with a small amount of co. Here we report a class of pd te hexagonal nanoplates hps with a pd20te7 phase. A proton exchange membrane is used in place of the electrolyte which allows h produced at the anode to migrate to the cathode. A second family of hydrogen cell vehicles is being developed to use gasoline or methanol fuel.
2h 2 g o 2 g 2h 2 o l electrode half equations higher. Common operating temperatures are in the range 50 120 c where high temperatures are usually pressurized dmfcs themselves are more efficient at high temperatures and. Methanol fuel cells can be used to power mobile phones. Fuel cell vehicle gasoline fuel fcvr.
Outline the function of the proton exchange membrane pem in the fuel cell. This fuel cell with fuel reformer fcvr extracts hydrogen from the hydrocarbon fuel. The methanol crossover effect in direct methanol fuel cells dmfcs can severely reduce cathodic oxygen reduction reaction orr performance and fuel efficiency. Efficiency of fuel cells based on g h for the formation of water from hydrogen and oxygen the efficiency of a hydrogen fuel cell should be nearly 80 corresponding to a cell potential of 1 23 v.
Methanol can also be used with hydrogen fuel cells. How would i work out the second one. Direct methanol fuel cells consume methanol ch 3 oh and oxygen to produce electricity heat carbon dioxide and water. As a result developing efficient catalysts with simultaneously high orr activity and excellent antipoisoning methanol capability remains challenging.
The system must produce hydrogen cleanly enough to avoid poisoning the fuel cell with carbon monoxide and fuel components. In tribology and interface engineering series 2003. Hence emf of the fuel cell is.